Rapid Technology Transfer

STRONG COLLABORATION FROM THE START
Since 2008, we have executed more than 200 successful product technology transfers. The key to this success is our strong partnership with our pharmaceutical and biotechnology customers. We offer the collaborative mindset and the scientific and technological problem-solving experience to ensure efficient and effective transfer of analytical and manufacturing processes from the start.

EFFICIENT & EFFECTIVE PROJECT MANAGEMENT
Before a partnership begins, it is important that a confidential disclosure agreement is in place and that the prospective customer issues a detailed request for proposal including specific project requirements. The Simtra BioPharma Solutions proposal responds to the technical considerations and lays out project assumptions, costs and timing. Once the project is awarded, contract negotiation starts with a collaborative kickoff meeting between customer and Simtra BioPharma Solutions at our facilities, resulting in a defined project scope and a comprehensive project plan.
Following contract approval, the project moves forward. Throughout this process, we bring to the table a team that includes a dedicated account executive, a seasoned project manager and experts representing the supply chain, R&D, technical services, quality, regulatory and sterility assurance functions.

A RESPONSIVE & EXPANSIVE SUPPLIER NETWORK
Our strong relationships with vendors allow for expedited purchase or production of necessary manufacturing equipment and supplies (such as tubing, filters and tanks) and product components. We are also able to use customer-supplied materials if desired.

READY SCIENTIFIC SUPPORT & ROBUST STANDARD PROCESSES
Our well-equipped R&D scientists are readily available to offer all necessary analytic services and to expedite any technical issues that may arise with product formulation, often acting as liaisons between our customers and our internal quality control teams during product transfer. They are also available to provide support post transfer. Manufacturing and technical team members collaborate on decision-making to achieve the best fit between product and process.
Our R&D team and our Lyophilization Center of Excellence are co-located with our manufacturing facility in Bloomington, Indiana, which streamlines the formulation and development process and ensures a smooth transition from laboratory to commercial scale. Our facility in Halle also has access to this expertise.
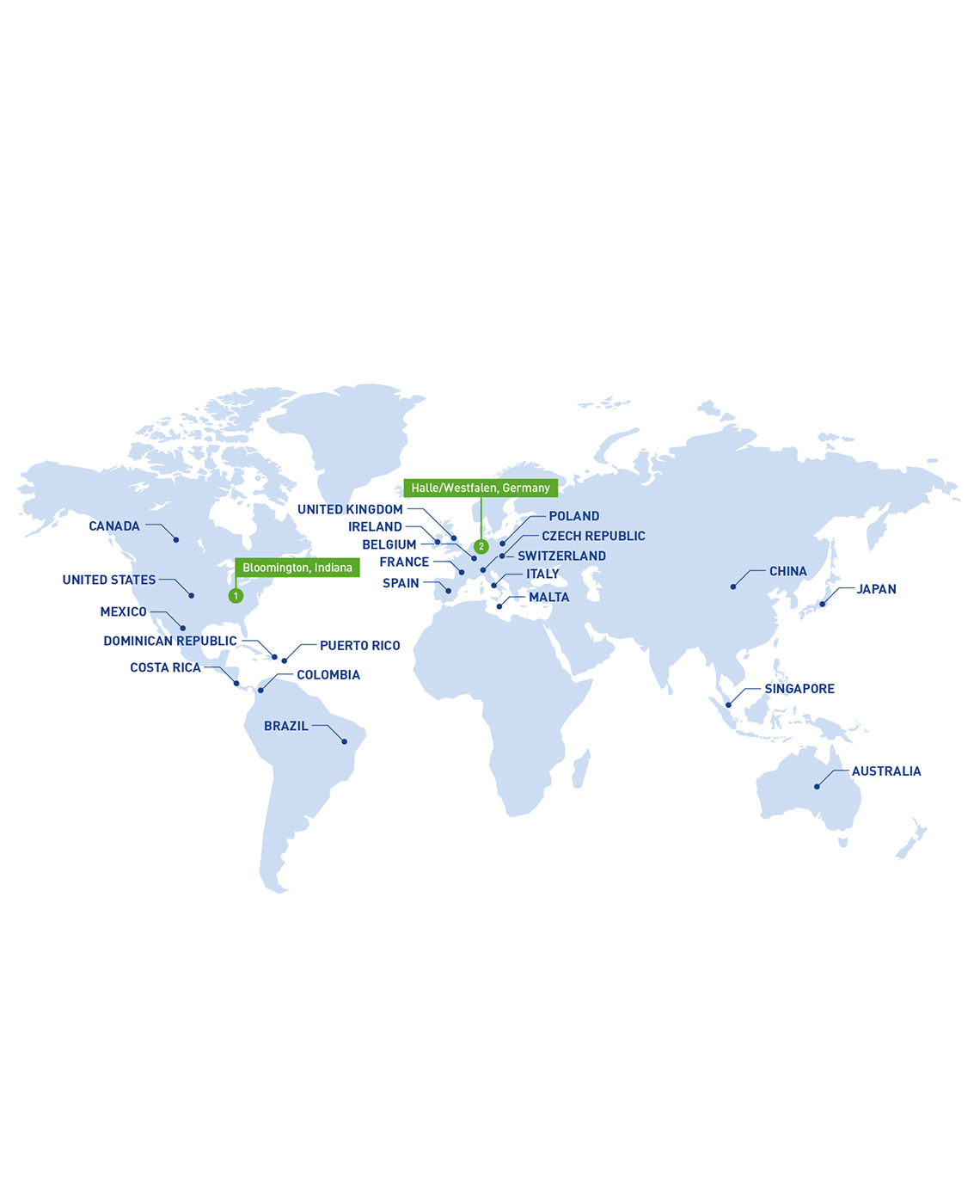
GLOBAL REGULATORY EXPERIENCE
We help clients manufacture products that reach more than 120 countries worldwide, so we have broad regulatory experience. Our facilities comply with FDA (United States), PDMA (Japan) and EMA (European Union) regulatory standards, and our global regulatory inspection history is strong. Our proven expertise in adhering to global regulatory requirements for quality control enables us to minimize compliance risks and potential production delays.
RELATED RESOURCES
Simtra Biopharma Solutions Capacity Update October 2023: Biologics
Simtra BioPharma Solutions (Simtra) is a premier CMO with a singular focus on sterile injectable drug product form/fill/finish services.
View resource

